Capnograph and Oximeter
Innovative and handheld capnograph and oximeter
FDA/Registration Number
64-2-2-2-0006971
PC-9000B
Features
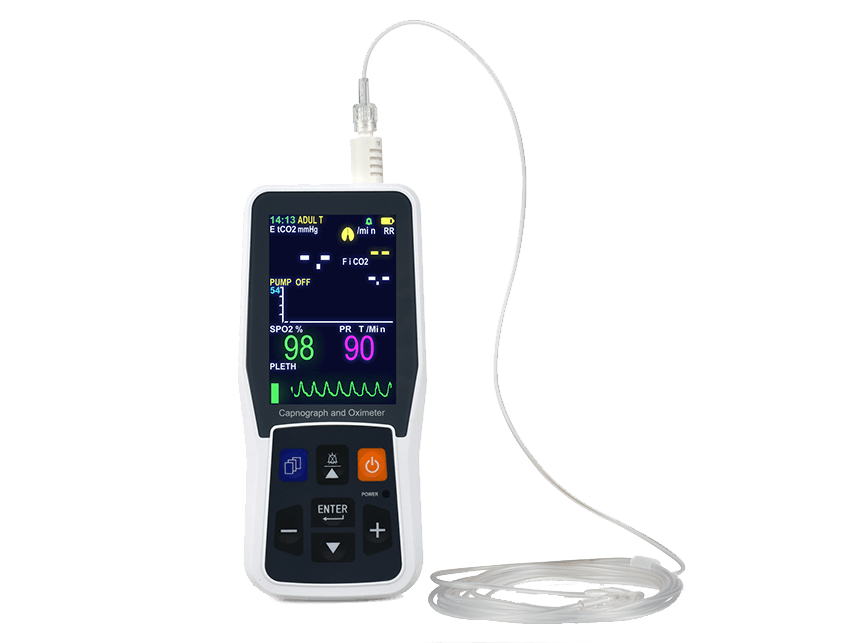
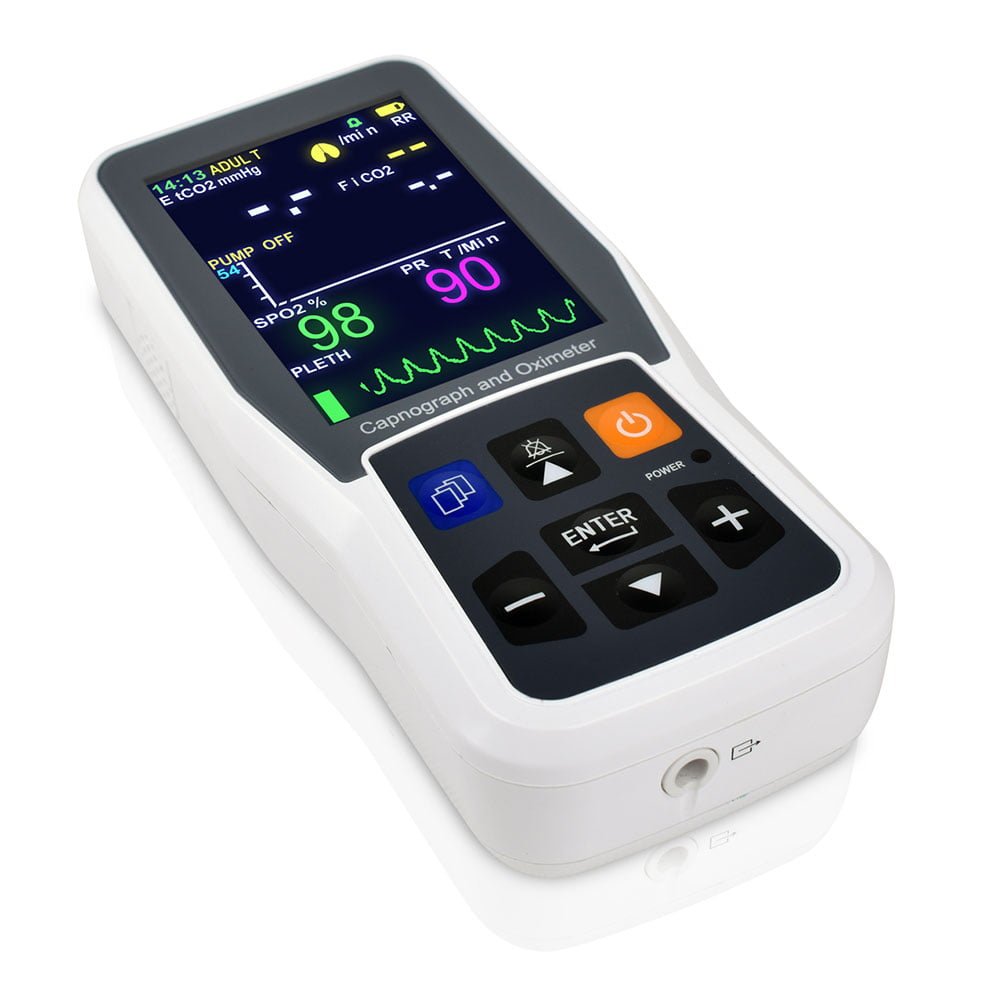
- 5 inch TFT display monitoring EtCO2,RR, SpO2 and PR
- Applicable for intubated or non-intubated patients
- Graphic trend storage up to 24 hours
- Data export to PC via USB cable for review and printout
- Language English/ Spanish/ Dutch

Precautions and Warnings
- This monitor is not MRI compatible and is not suitable for use within the magnetic field during the operation of MRI or CT. However, the sample lines supplied alongside the unit by the distributor are MRI compatible and may be extended into the MR or CT field. In this case, the monitor must remain outside of the room.
- The use of accessories and cable other than those specified, with the exception of cables sold by the manufacturer of the device as replacement parts for internal component, may result in increased emissions or decreased accuracy of the device.
- Only use manufacturer designated accessories to ensure compliance with appropriate standards
- It is not allowed to remove the cover of the monitor.
- This monitor provides concentration of EtCO2, respiration rate, oxygen saturation and pulse rate. This data only provides assistance for diagnosis and actual diagnosis shall be made by suitably qualified clinical staff using all the clinical information and symptoms.
- In order to prevent pressure sores and correct circulation the SpO2 sensor must be repositioned regularly, depending on the type of sensor used.
Notice the warnings on the label and accompanying documents before use
Distributed by
Alcotec International Co., Ltd
77/152 Sinn Sathorn Tower 35th Fl., Krungthonburi Road,
Klongtonsai, Klongsan, Bangkok 10600. Tel. 02-862-0959
ฆพ. 846/2568
Capnograph Oximeter PC9000B Specification Sheet
RELATED PRODUCTS
Need more details for each of the product?
OUR CUSTOMERS