FDA/Registration Number
64-2-2-2-0005879

UP-7000
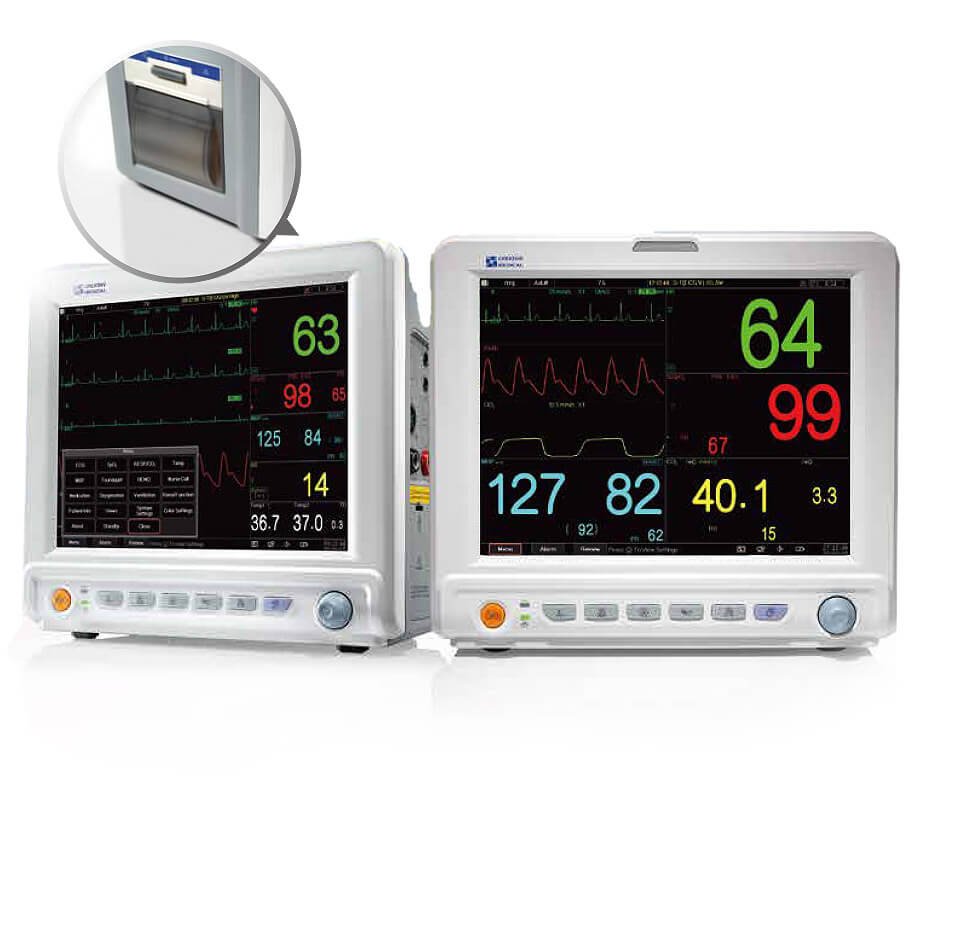
Features
- Color display 12.1”
- Up to 9 waveforms simultaneously display
- Monitor patient’s physiological parameters including ECG, heart rate (HR), non-invasive blood pressure (NIBP), oxygen saturation (SpO2), Pulse rate (PR) and temperature
- Waveform freezing and S-T segment measurement is available
- Supports the addition of internal blood pressure (IBP) and end-tidal carbon dioxide (EtCO2) measurement (Optional)
- Built-in rechargeable battery

Precautions and Warnings
- WARNING for PACEMAKER PATIENTS: Although the pacemaker pulse inhibition function is available in this device, the heart rate meter may continue to count the pacemaker rate during occurrences of cardiac arrest or some arrhythmias. Do not rely entirely upon rate meter ALARMS. Keep pacemaker patients under close surveillance. See this manual for disclosure of the pacemaker pulse rejection capability of this instrument.
- Monitoring a single person at a time.
- Check SpO2 probe application site periodically (every 30 minutes) to determine circulation, positioning and skin sensitivity.
- Do not immerse the monitor or its accessories in liquid to clean.
Notice the warnings on the label and accompanying documents before use
Distributed by
Alcotec International Co., Ltd
77/152 Sinn Sathorn Tower 35th Fl., Krungthonburi Road,
Klongtonsai, Klongsan, Bangkok 10600. Tel. 02-862-0959
ฆพ. 846/2568
Patient Monitor UP7000 Specification Sheet
RELATED PRODUCTS
Need more details for each of the product?
OUR CUSTOMERS